It is great to see you here. To see all contents of the website, please register or restore your access.
We believe you will come back gladly.
GeneProof Team
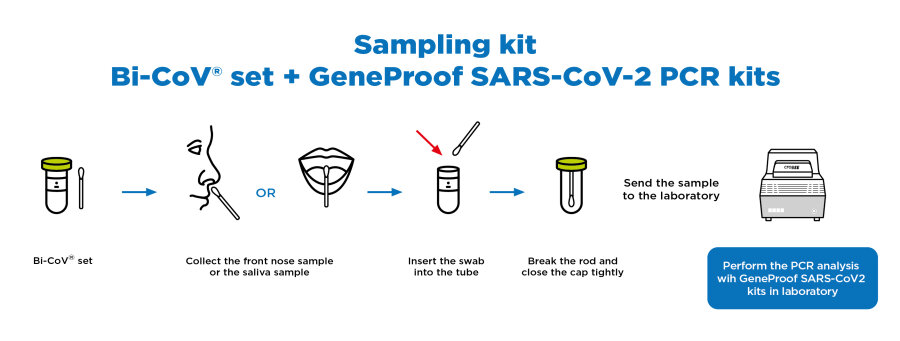
Especially for massive screening testing of public (i.e. mandatory testing of company employees who can collect the samples themselves), we offer a solution based on the sample collection from the front nose or mouth (saliva sample) using the sampling kit Bi-CoV® (Bioinova, s.r.o.) that has been validated for use with GeneProof PCR kits without the need of RNA extraction.
The sampling kit is comprised of a swab and a tube with the medium. The sample collection is done by a standard swab from the front nose or the oral cavity (tongue radix area). The sample collection is very easy and safe. The material collected consists of nose mucus or saliva and secretions from the upper respiratory tract. This mix is ideal for the detection of virus. The virus is inactivated and RNA that is released in the medium is also stabilized during the transport into a laboratory. The standard PCR analysis is performed in the laboratory with GeneProof PCR kits that have been optimized and certified for this type of sample collection (clinically validated).
When sampling from the front nose, the test person first blows his/her nose to move the sampling material from the nasopharynx to the front nose. Then the sampling rod is inserted into the front part of the nose (ca. 2 cm deep) and rotates three times in each direction.
When sampling from the oral cavity (saliva), the test person should not eat, drink, brush his teeth, rinse his mouth, smoke or use chewing gum for at least 30 minutes before sampling. First, it is necessary to cough properly into the closed mouth so that the sampling material from the throat and respiratory tract reaches the oral cavity. The tampon is then inserted into the place of accumulated saliva and mucus (on the tongue, under the tongue, near the molars, etc.), eventually wipe around the palatoglossal arches.
You do not need to isolate the viral RNA, therefore the procedure is significantly shorter, easier, and cheaper.
Order information for 100 examinations:
| Product | REF | Packaging | Number |
|
Bi-CoV® Set |
BI001-50GP |
50 pcs | 2x |
| GeneProof SARS CoV-2 Screening PCR Kit |
COV2S/GP/100 |
100 rxn | 1x OR |
| GeneProof SARS CoV-2 PCR Kit |
COV2/GP/100 |
100 rxn | 1x |
+420 543 211 679
sales@geneproof.com
Send request
+420 730 176 222
support@geneproof.com