It is great to see you here. To see all contents of the website, please register or restore your access.
We believe you will come back gladly.
GeneProof Team
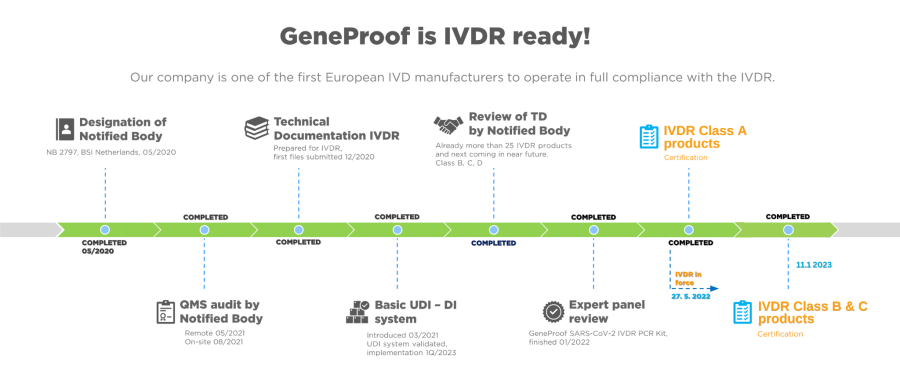
GeneProof is among the first European IVD manufacturers to fully comply with the in vitro Diagnostic Regulation (IVDR)
As of 27 May 2022, the company's activities are fully compliant with Regulation (EU) 2017/746 of the European Parliament and of the Council, as amended.
Our entire portfolio of more than 80 CE IVD products are available under the new EU IVD rules.
All Class A products are now IVDR certified:
Get the new EC Declaration of Conformity and Instruction for Use are available on our product pages.
All other PCR kits (which were classified as other IVD devices according to the IVDD) are fully compliant with the REGULATION (EU) 2022/112 – (IVDR amendment of 01/2022) and are fully available.
Nevertheless, we successfully continue on our way to IVDR certification of our D Class devices:
You can count on GeneProof – today and in the future!
Do you want to ask more? Do not hesitate to contact us on support@geneproof.com.
+420 543 211 679
sales@geneproof.com
Send request
+420 730 176 222
support@geneproof.com
